Amid the rising demand for innovative healthcare solutions for pets, Chinese Pet Owners Feed COVID Pills to Pets – an unconventional method to treat a deadly feline disease.
By repurposing human COVID-19 antiviral medications, they have successfully tackled feline infectious peritonitis (FIP), a fatal disease caused by a coronavirus specific to cats. This unexpected use of COVID pills is gaining widespread attention and reshaping how pet owners view animal healthcare in post-pandemic China.
A New Lease on Life for Cats with FIP
Feline infectious peritonitis is a severe and often fatal condition caused by the feline coronavirus, which attacks white blood cells and triggers inflammatory reactions throughout the body. Until recently, FIP was considered untreatable, leaving pet owners helpless as their cats succumbed to the disease.
However, the discovery of the effectiveness of certain human COVID-19 antiviral drugs, like Lagevrio by Merck & Co, has brought newfound hope to cat owners.
Lagevrio, originally designed to combat COVID-19 in humans, is being used off-label to treat cats with FIP. Pet owners report remarkable success stories, with cats recovering from a disease that was once a death sentence.
Social media platforms like Xiaohongshu, often dubbed China’s Instagram, are brimming with testimonies from pet owners sharing their experiences and urging others to adopt this treatment for their furry companions.
Read : China Declares State of Emergency Over HMPV Virus Outbreak
One Xiaohongshu user recounted, “COVID-19 drugs for humans saved my cat’s life. I hope my notes help more people save their furry babies and reduce their suffering.”
Read : Covid-19 Still Kills 1,700 a Week Around the World: WHO
The affordability of human COVID drugs compared to specialized veterinary treatments has further fueled their popularity, providing a practical alternative to the black-market procurement of the otherwise prohibitively expensive antiviral GS-441524.
The Cost Barrier and Black-Market Challenges
One of the primary reasons for this trend is the cost disparity between human COVID drugs and veterinary-specific treatments. GS-441524, a promising antiviral for FIP, is not approved by the US FDA and is often sourced through informal networks at exorbitant prices. Chinese pet owners frequently pay tens of thousands of yuan for a single course of GS-441524, only to encounter fake versions or diluted dosages.
In contrast, human COVID-19 antivirals like Lagevrio are far more affordable and accessible. A 40-pill bottle of Lagevrio costs approximately 1,725 yuan ($235) and is sufficient to treat more than one cat.
Homegrown Chinese pharmaceutical companies, such as Henan Genuine Biotech Co, Simcere Pharmaceutical Group, and Shanghai Junshi Biosciences, have also developed similar medications that offer competitive pricing, further expanding the options available to pet owners.
This affordability allows more pet owners to access effective treatments, sparing them from the financial strain of veterinary-specific alternatives.
Additionally, many cat owners have turned to human nutritional supplements for their pets, citing the high costs of veterinary products. A Xiaohongshu user highlighted this sentiment, saying, “I don’t understand why medicines for pets are so expensive. You just need to adjust the dosage of human medicines if you are going to use them on cats.”
A Controversial Practice with Ethical and Regulatory Implications
While the use of human medications for pets has garnered significant attention and success stories, it has also raised ethical and regulatory questions.
Merck & Co, the manufacturer of Lagevrio, has stated that it has not tested the drug on cats and has no plans to do so. This leaves the responsibility of dosage adjustment and administration entirely on pet owners, a risky undertaking given the potential for adverse reactions in animals.
The practice contrasts sharply with earlier controversies, such as the use of ivermectin—a drug for treating parasitic worms in animals—by humans during the pandemic.
Regulatory bodies like the US FDA had to issue strong warnings against such uses, emphasizing the risks of administering animal medications to humans. Similarly, experts warn of the potential dangers of repurposing human drugs for animals without proper veterinary guidance.
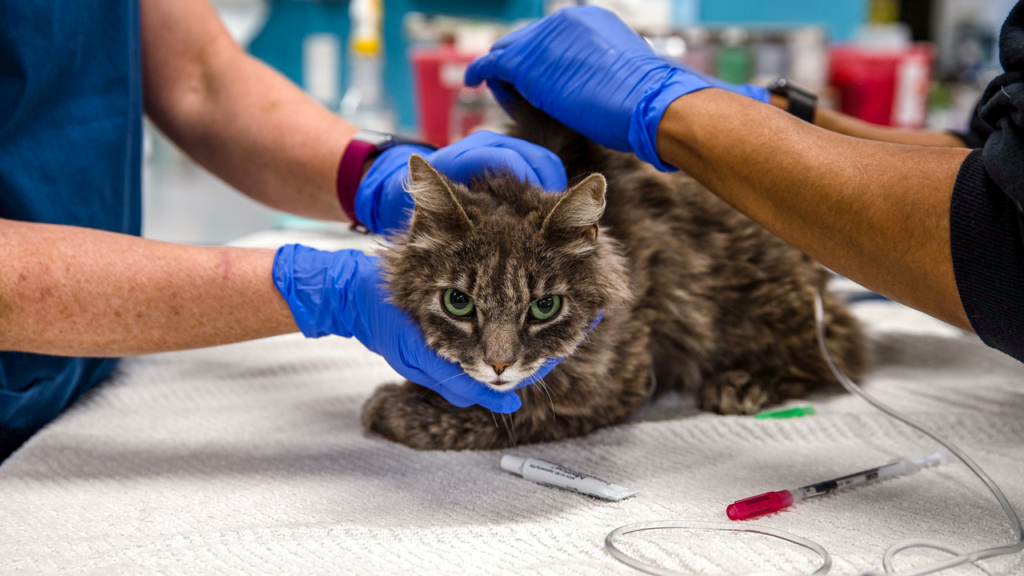
Despite these concerns, the desperation of pet owners and the lack of affordable veterinary alternatives have driven this trend. FIP’s unique characteristics—it is non-contagious to humans, dogs, or other animals—add a layer of reassurance for those using these drugs.
However, as the practice becomes more widespread, there is an urgent need for regulated, accessible, and affordable treatments tailored specifically to animals.
The innovative use of human COVID-19 antiviral drugs by Chinese pet owners to treat feline infectious peritonitis showcases the lengths to which people are willing to go to save their beloved pets.
While this approach has provided life-saving solutions for many cats, it also highlights the pressing need for more accessible veterinary treatments. As this trend gains traction, it underscores the importance of balancing innovation with safety and regulation in the evolving landscape of pet healthcare.