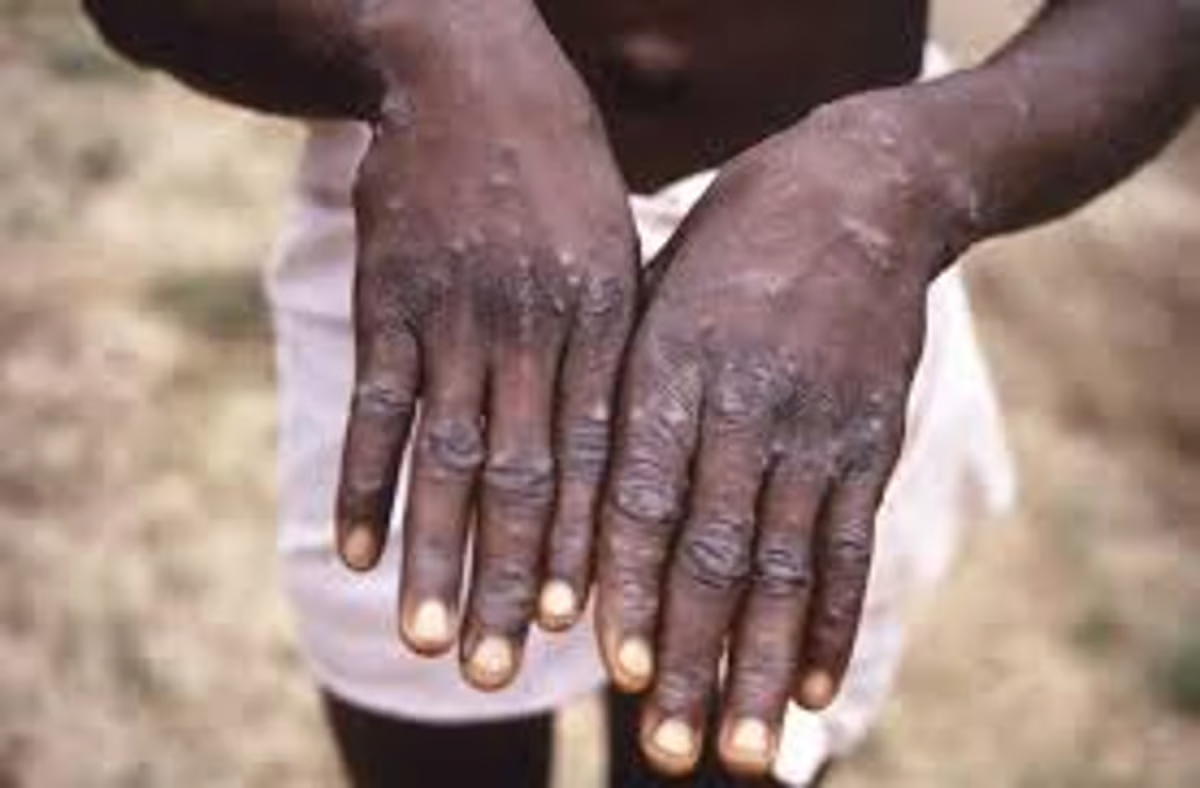
WHO – The World Health Organization has taken a pivotal step in the fight against mpox by approving the MVA-BN vaccine for limited use. This vaccine, developed by Bavarian Nordic A/S, is now the first mpox vaccine to be prequalified by the WHO, making it a crucial tool in controlling mpox outbreaks, particularly in Africa.
The approval represents a significant advancement in global health, addressing the urgent need for effective prevention measures against this virus.
The MVA-BN vaccine, which requires two doses administered four weeks apart, has demonstrated up to 82% effectiveness in preventing mpox. This high level of efficacy is a promising development in the fight against the disease, providing a much-needed option for countries experiencing outbreaks.
The WHO’s endorsement of this vaccine highlights its commitment to improving access to life-saving interventions, especially in regions where they are most needed.
WHO Prequalifies First Mpox Vaccine
The inclusion of the MVA-BN vaccine on the WHO’s prequalification list is a landmark achievement. This prequalification is essential for ensuring that vaccines meet international standards of quality, safety, and efficacy. By adding the MVA-BN vaccine to its list, the WHO is facilitating greater access to this critical tool for combating mpox.
The decision follows a comprehensive review by the European Medicines Agency, which assessed the vaccine’s safety and effectiveness.
Read : 20 Crore Indians Living Inactive Lives in Accordance with WHO Standard
Read : Covid-19 Still Kills 1,700 a Week Around the World: WHO
The WHO’s prequalification ensures that the vaccine will be available to communities in need, particularly in Africa, where mpox outbreaks have been a pressing concern. This step is expected to enhance the global response to the disease and support efforts to control its spread.
Focus on African Outbreaks
The approval of the MVA-BN vaccine is especially significant for Africa, where mpox outbreaks have been particularly severe. WHO Director-General Dr. Tedros Adhanom Ghebreyesus emphasized the importance of equitable access to the vaccine in tackling these outbreaks.
He stressed the need for a swift scale-up in procurement and distribution to ensure that the vaccine reaches those who need it most.
“This first prequalification of a vaccine against mpox is an important step in our fight against the disease, both in the context of the current outbreaks in Africa and in future,” Dr. Ghebreyesus stated.
The WHO’s focus on ensuring equitable access underscores its commitment to addressing global health disparities and improving health outcomes for vulnerable populations.
The MVA-BN vaccine is expected to play a critical role in preventing infections, stopping transmission, and ultimately saving lives. As Dr. Ghebreyesus noted, the vaccine is a valuable addition to the public health toolkit, complementing other measures in the fight against mpox.
Vaccine Efficacy and Administration
The MVA-BN vaccine has shown promising results in clinical trials, with an estimated 82% effectiveness in preventing mpox after the administration of both doses. This level of efficacy is a significant achievement, reflecting the vaccine’s potential to provide robust protection against the virus.
Administered in two doses four weeks apart, the MVA-BN vaccine is intended for use in individuals over the age of 18. The two-dose regimen ensures that recipients receive optimal protection, enhancing the vaccine’s effectiveness in preventing mpox. This approach aligns with standard vaccination practices, where multiple doses are often required to achieve full immunity.
The approval of this vaccine is a crucial development in the global response to mpox, offering a new tool for public health authorities to combat the disease. As the vaccine becomes available in selected countries, efforts will be focused on ensuring that it is distributed effectively and reaches the populations most at risk.
In conclusion, the WHO’s approval of the MVA-BN vaccine represents a major milestone in the fight against mpox. By adding the vaccine to its prequalification list, the WHO is paving the way for improved access to this critical intervention, particularly in regions experiencing severe outbreaks.
The high efficacy of the vaccine and the WHO’s emphasis on equitable distribution highlight the importance of this development in advancing global health.