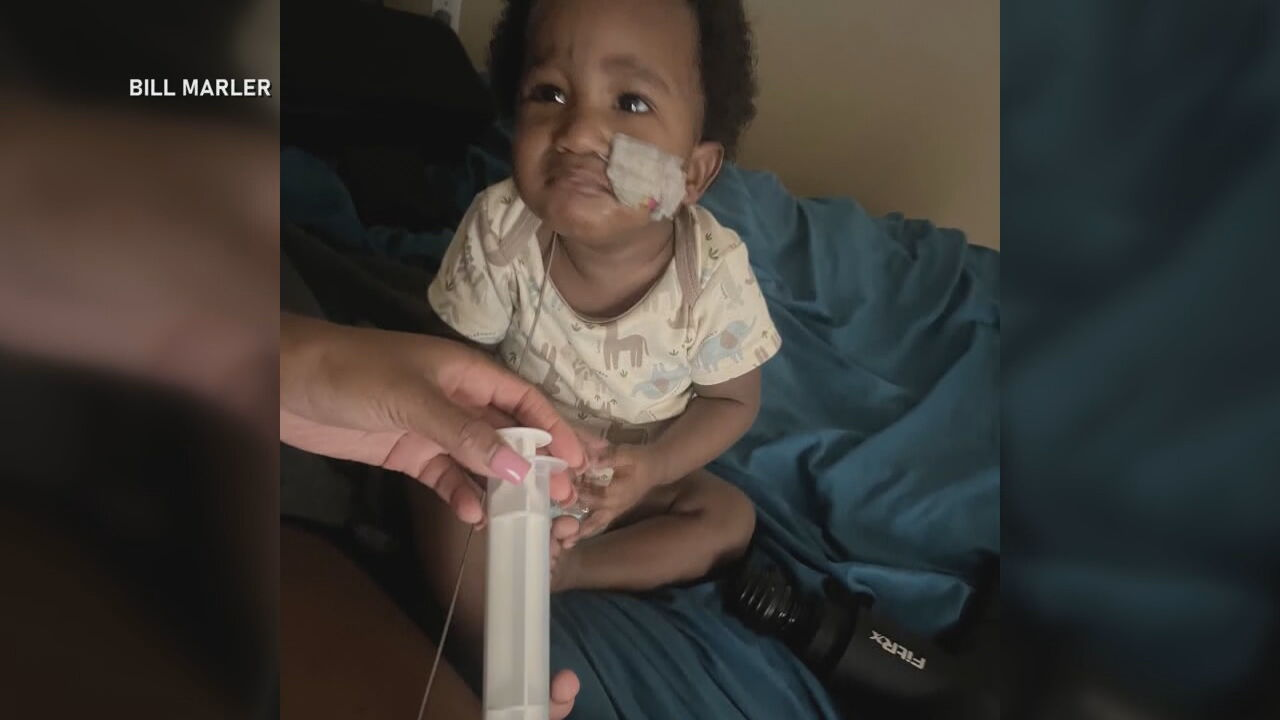
A 10-month-old boy in Portland, Oregon, is continuing a difficult medical battle after being diagnosed with infant botulism, a rare but potentially life-threatening condition, following consumption of contaminated ByHeart baby formula. Ashaan Carter’s case has drawn national attention amid a sweeping recall of all ByHeart formula products and mounting questions over how the recalled formula reached some of the country’s most vulnerable families. His illness is part of a broader outbreak that has sickened more than 50 infants across the United States and has triggered lawsuits, federal inspections, and renewed scrutiny of formula donation programs operating during and after the nationwide infant formula shortage.
Ashaan’s mother, Angel Carter, said she received a can of ByHeart formula in early November through a state-connected assistance effort intended to support low-income and homeless families. The formula was provided just days before a national recall was issued. Carter, who had been exclusively breastfeeding, accepted the formula after being told it was close to breast milk and suitable as her milk supply began to diminish. Within days of consuming it, Ashaan’s health began to deteriorate rapidly, setting off a chain of hospitalizations and long-term complications that continue months later.
How Contaminated Formula Reached Vulnerable Families
According to Carter, the ByHeart formula was provided by an Oregon Department of Human Services case worker as part of food and housing support she receives from the state. While state officials declined to comment on her specific case, they acknowledged that the agency had received ByHeart formula from PDX Diaper Bank, a Portland-based nonprofit that distributes diapers, formula, and other essentials to families in need.
PDX Diaper Bank was one of nearly two dozen nonprofit organizations nationwide involved in ByHeart’s “OpenHearted Initiative,” a program the company said was designed to donate formula to families experiencing hardship. ByHeart has stated that since June 2022, nearly 24,000 cans of its baby formula were distributed through nonprofit partners to groups aiding homeless and vulnerable families.
These donations occurred during a period marked by severe formula shortages across the United States, when families and aid organizations were scrambling to secure infant nutrition. However, all ByHeart products have since been recalled after the discovery of potential contamination linked to cases of infant botulism. The recall applies to formula produced since the company began manufacturing in March 2022, effectively halting all sales and distribution.
PDX Diaper Bank’s executive director, Rachel Alston, said the organization received approximately 400 cans of donated ByHeart formula through Baby2Baby, a Los Angeles-based nonprofit known for distributing essential goods to families in need and supported by numerous high-profile public figures. More than 300 cans were distributed to partner organizations before the recall was announced. Alston said that once the recall was issued, partners took immediate steps to notify families and pull remaining products from circulation.
Read : Langkawi Island, Malaysia: A Tropical Paradise Awaits
Carter said she received a text message from her case worker warning her to stop using the formula after the recall became public. By that time, however, Ashaan had already fallen seriously ill. The case has intensified scrutiny of how donated formula is tracked, monitored, and recalled within decentralized charity networks, particularly when products are routed through multiple organizations before reaching families.
Ashaan Carter’s Medical Crisis and Ongoing Recovery
Soon after consuming the ByHeart formula, Ashaan developed severe constipation, one of the earliest signs of infant botulism. His condition progressed quickly to include profound muscle weakness. Carter said her son became so limp that he could not lift or support his head. Alarmed by his sudden decline, she brought him to an emergency room, where he was eventually transferred to Randall Children’s Hospital in Portland for specialized care.
Doctors diagnosed Ashaan with presumed infant botulism, a condition caused by exposure to Clostridium botulinum spores, which can produce a potent neurotoxin in an infant’s intestines. The diagnosis was linked to the contaminated formula, according to Dr. Sylvia Peterson-Perry, a family medicine physician who delivered Ashaan at birth and continues to care for both him and his mother. Infant botulism is rare but dangerous, often requiring intensive treatment and prolonged recovery.
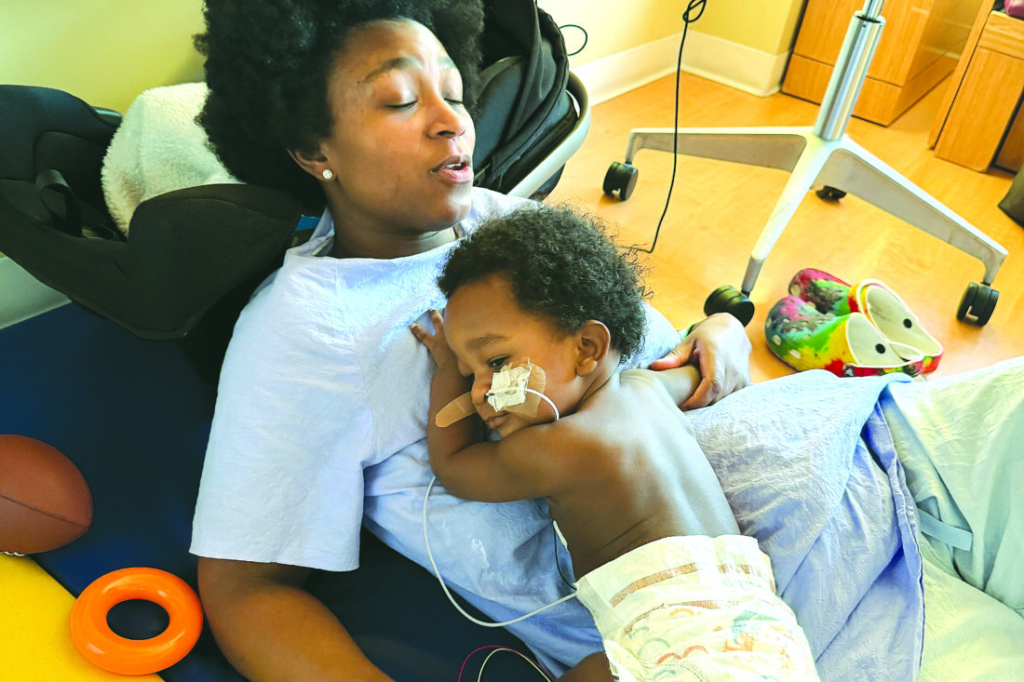
Ashaan was treated with BabyBIG, an intravenous medication containing antibodies that neutralize the botulinum toxin and prevent further nerve damage. While the treatment can halt progression of the disease, recovery depends on the gradual regeneration of affected nerves, a process that can take weeks or months. Ashaan remained hospitalized for nearly two weeks in November before being discharged without a feeding tube.
However, his condition soon worsened. Carter said her son experienced dramatic weight loss and increasing weakness after returning home. In December, he was hospitalized again, this time in a more critical state. Carter said she feared he might not survive as his health declined rapidly.
Doctors reinserted a feeding tube after determining that Ashaan’s weakened muscles made it unsafe for him to feed normally. According to his physician, it remains unclear how long the tube will be necessary. Ashaan is now undergoing therapy to relearn basic developmental skills, including crawling and speaking, which were disrupted by the illness. His recovery remains ongoing, with careful monitoring and support required to address lingering neurological and muscular effects.
Investigations, Lawsuits, and Broader Implications
The outbreak linked to ByHeart formula has prompted investigations by federal agencies and a growing wave of litigation. The U.S. Centers for Disease Control and Prevention reported that no new cases of infant botulism tied to the outbreak have been identified since December 17. The U.S. Food and Drug Administration has conducted inspections at ByHeart’s production facilities but has not publicly disclosed details about the source of the contamination. Production at the company remains shut down.
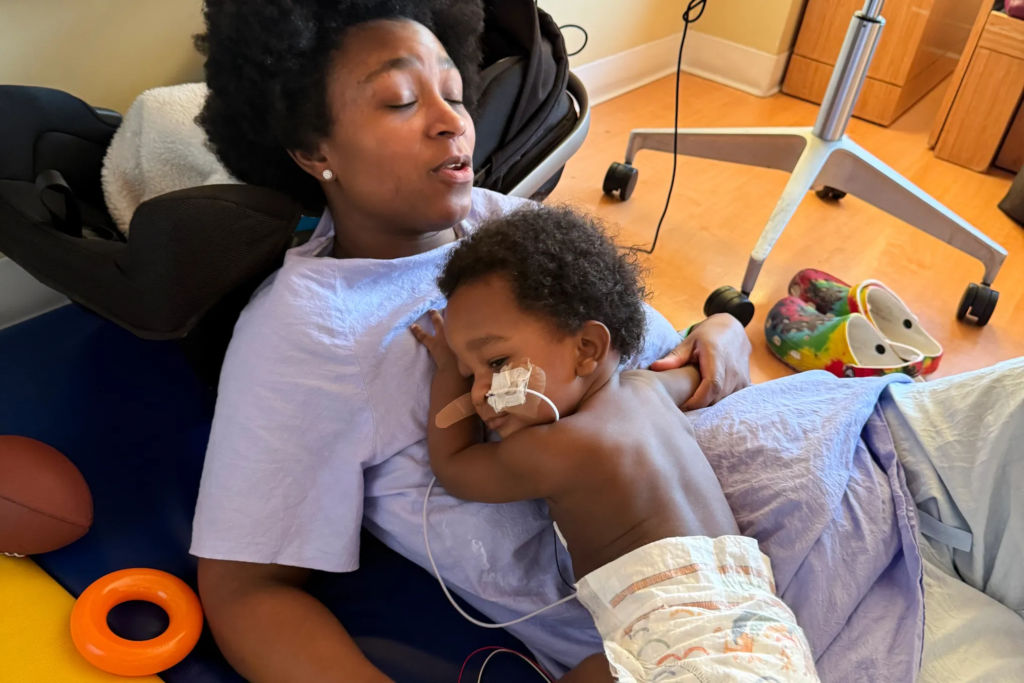
Legal action is advancing on multiple fronts. Seattle-based food safety attorney Bill Marler said he represents more than 30 families whose infants were sickened by ByHeart products. At least 18 lawsuits have been filed against the company and retailers that sold or distributed the formula. This week, Marler asked a federal panel to consolidate the cases in a U.S. district court in New York, a move that could streamline pretrial proceedings and centralize evidence related to the outbreak.
ByHeart has stated that it worked with Baby2Baby and other nonprofit partners to ensure recalled products were removed from circulation and that families were notified as quickly as possible. Baby2Baby has not responded to media requests for comment. Healthbeat, a nonprofit news outlet focused on public health reporting, was the first to report that recalled ByHeart formula had been distributed to organizations serving at-risk families.
Medical professionals and advocates have emphasized the broader implications of Ashaan’s case, particularly for families who rely on donated or state-supported food assistance. Dr. Peterson-Perry described the situation as devastating for vulnerable families who trust that donated products are safe and that social services systems will protect their children’s health. The case has renewed calls for stricter oversight of formula manufacturing, clearer accountability within donation networks, and faster communication when recalls involve products distributed outside traditional retail channels.
As Ashaan Carter continues his recovery, his case stands as one of the most detailed accounts of how the ByHeart outbreak affected individual families. It also highlights the intersection of public health, corporate responsibility, and social services during a period when many parents had few alternatives for feeding their infants. The legal and regulatory outcomes of the outbreak are still unfolding, while affected families face long-term medical, emotional, and financial consequences.